Symphony® X Peptide Synthesizer
Maximum flexibility for research, optimization and production
Software designed for 21CFR part 11 compliance
Enabling traceability through audit trail and reporting functions for efficient reviews
User management including password management support and login rules
Electronic signature in compliance with 21 CFR Part 11
Symphony® X
MULTIPLEX PEPTIDE SYNTHESIZER
Purity
Productivity
Power
Enabling Traceability
Symphony® X instruments in cGMP facilities are used for synthesis of peptides required in applications, such as clinical studies and neoantigen trials as well as cosmetic formulations. For instrumentation in these environments, safe and accurate data storage and traceability are required. Regulatory agencies such as the United States Food and Drug Administration (FDA) and European Medicines Agency require certain controls and documentation for software involved in the processing of electronic data. Title 21
CFR Part 11 is the part of the Code of Federal Regulations that establishes FDA regulations on electronic records and electronic signatures, while EU Annex 11 provides similar guidance in Europe.
To help address these requirements, Symphony X software includes features that enable traceability via the following functions, for efficient reviews:
User Management
Audit Trail
Data Integrity
Electronic Signatures
21 CFR part 11 compliance and IQ/OQ
In combination with training by qualified personnel, a comprehensive quality management system, and appropriate documentation including Installation Qualification and Operation Qualification (IQ/OQ), software designed for 21 CFR Part 11 compliance helps Symphony X users meet and exceed regulatory standards.
IQ/OQ support services verify and document that your instrument is supplied, installed, and operating according to GPT specifications. GPT-certified field service engineers inspect critical components, ensure that your systems function properly and reproducibly during automated synthesis operations, and provide documentation to help you meet regulatory requirements.

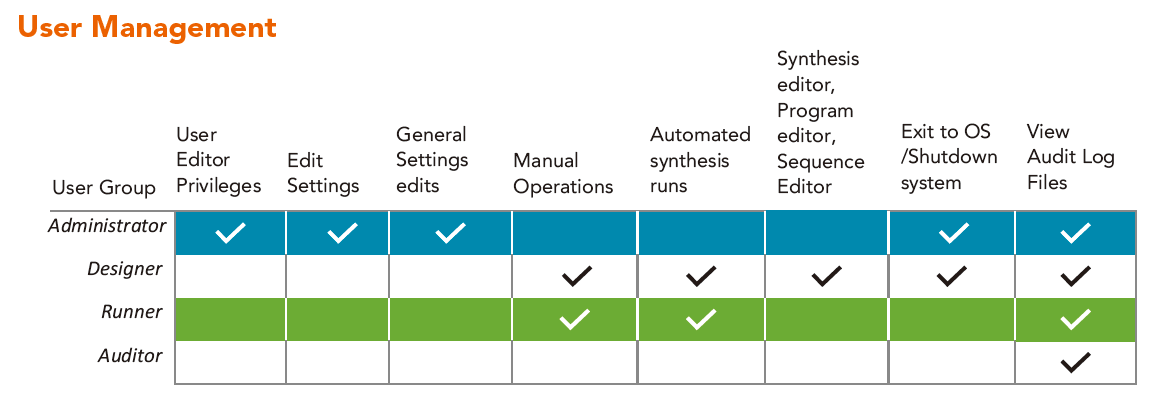
User Management
Administrators can configure passwords by defining password length, complexity, frequency of change, and exclusion of used passwords. Administrators may also define the maximum number of failed login attempts. After a specified (and configurable) period of inactivity, users are automatically logged out and must re-enter a password to log in to the software.
At a minimum, a password is required when creating a new or editing an existing program or sequence file. Beyond this, more stringent controls can be assigned by administrators.
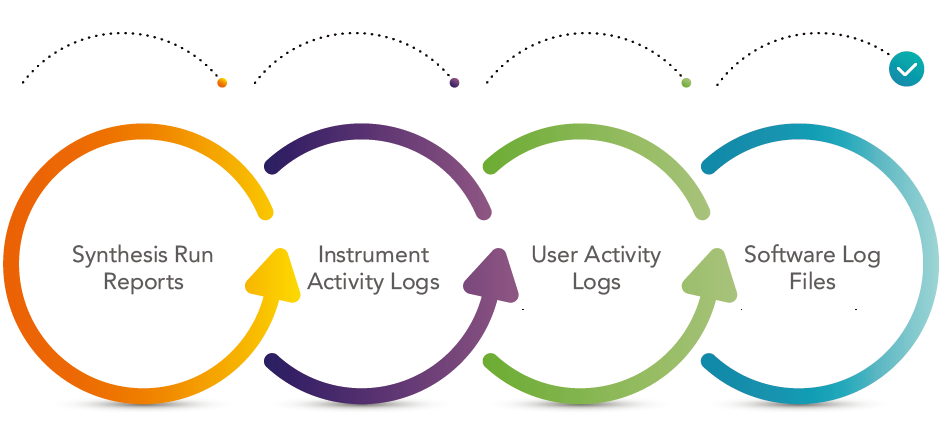
Audit Trail
An audit trail is a time-stamped, modification-protected electronic data file detailing all system events and record modifications. Symphony X software allows a user or auditor to view synthesis run reports, instrument activity log files, user activity log files, and software log files. It is possible to export and print the full contents of all records including the audit trail.
Detailed records of user and software activity as well as data from internal sensors monitoring fluid deliveries, heating, etc. also make these files useful diagnostics for instrument performance and troubleshooting.
Data Integrity
Synthesis program, cleavage, and sequence files are protected and encrypted by document passwords and recorded as digitally signed PDFs using PDFTron technology.
A backup, archiving and recovery strategy is necessary in order to ensure that the reconstruction of data is achievable in the unexpected event of data loss. Symphony X software allows users to create a backup (restore point) on local or external drives, from which they can effectively restore the software.

Electronic Signatures
Electronic signatures function in the same fashion as hand-written signatures. To meet FDA requirements regarding the use of electronic signatures, the software must employ at least two distinct identification components such as an identification code and password. When a new user is added to the system, they are confirmed to have a unique username, username-password combination, email, and legal name that is used for Digital Signatures.
Completed synthesis runs are signed off by the user who initiated the synthesis. This is manifested in the synthesis run report as a digital signature. Signed synthesis reports contain the printed name of the signer, date, time when the signature was executed, and the reason for signing.
Audit Support Documentation
In addition to training records, feature, and user guides, an audit support document can be provided that explains in detail how individual software features align with the specific requirements outlined in 21 CFR Part 11.
Symphony X
SYSTEM SPECIFICATIONS
Number of reaction vessels:24 (12 with pre-activation)
Synthesis scale range:0.005* mmol - 24 mmol (up to ~2 g of resin per RV) *minimum deliveries 1 mL or 0.5 mL with Single-Shot
Reaction vessel volume:Plastic, disposable - 10 mL, 45 mL;Borosilicate glass - 10 mL, 40 mL
Number of solvent positions:1 primary solvent, 20 L capacity. Other sizes available
7 user-defined solvent positions, 0.5 L - 4 L capacities. Other sizes available
Number of amino acid positions:Up to 40 amino acid positions 10 mL, 120 mL and 400 mL bottles
Chemistries supported:Fmoc, t-Boc, organic, peptoid, combinatorial, branched, PNA
Activation:DIC/HOBt, DIC/OxymaPure, HBTU, HATU, HCTU, TBTU, PyBOP and others
Throughput:User defined
Fluid transfer method:Positive pressure with nitrogen
Agitation method:Nitrogen bubbling and/or oscillation mixing can be adjustable and programmable
Otional heating method:Induction heating with IR pyrometer temperature sensinge
Cleavage:Automatic, programmable (for Fmoc chemistry only)
Waste container:(2) 20 L D.O.T rated containers with over-flow sensor in cap
Reporting:Real-time log file updated for every instrument function, print to external file, pull up on screen or printer
Power:115 V/60 Hz or 230 V/50 Hz
Dimensions:45”W, 29”D, 66”H (114 cm W, 74 cm D, 168 cm H)
Weight:550 lbs. (250 kg)
Warranty:One year, parts and labor
Optional:Infrared (IR) Heating UV monitoring





 TEL:626-887-5831,909-990-7728
TEL:626-887-5831,909-990-7728 Email:ussales@molecular-lifesciences.com,info@molecular-lifesciences.com
Email:ussales@molecular-lifesciences.com,info@molecular-lifesciences.com