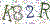Symphony® X多肽合成仪
Maximum flexibility for research, optimization and production
研究,优化和生产的最大灵活性
Software designed for 21CFR part 11 compliance
Enabling traceability through audit trail and reporting functions for efficient reviews
User management including password management support and login rules
Electronic signature in compliance with 21 CFR Part 11
软件设计为21CFR第11部分合规
启用可追溯性通过审计跟踪和报告功能,以便进行有效的审查。
用户管理,包括密码管理支持和登录规则
电子签名符合21 CFR Part 11
Symphony® X
MULTIPLEX PEPTIDE SYNTHESIZER
多路复用多肽合成仪
Purity
Productivity
Power
纯度
生产力
能力
Enabling Traceability
实现可追溯性
Symphony® X instruments in cGMP facilities are used for synthesis of peptides required in applications, such as clinical studies and neoantigen trials as well as cosmetic formulations. For instrumentation in these environments, safe and accurate data storage and traceability are required. Regulatory agencies such as the United States Food and Drug Administration (FDA) and European Medicines Agency require certain controls and documentation for software involved in the processing of electronic data. Title 21
CFR Part 11 is the part of the Code of Federal Regulations that establishes FDA regulations on electronic records and electronic signatures, while EU Annex 11 provides similar guidance in Europe.
Symphony®X仪器在cGMP设施中用于合成应用所需的多肽,如临床研究和新抗原试验以及化妆品配方。 对于这些环境中的仪器,需要安全准确的数据存储和可追溯性。 监管机构如美国食品和药物管理局(FDA)和欧洲药品管理局要求对涉及处理电子数据的软件进行某些控制和文档。 标题21 CFR Part 11是联邦法规法典的一部分,建立了FDA关于电子记录和电子签名的法规,而EU Annex 11在欧洲提供了类似的指导。
To help address these requirements, Symphony X software includes features that enable traceability via the following functions, for efficient reviews:
User Management
Audit Trail
Data Integrity
Electronic Signatures
为了帮助解决这些需求,Symphony X软件包含了通过以下功能来实现可追溯性的功能,以便进行有效的审查:
用户管理
审计跟踪
数据完整性
电子签名
21 CFR part 11 compliance and IQ/OQ
In combination with training by qualified personnel, a comprehensive quality management system, and appropriate documentation including Installation Qualification and Operation Qualification (IQ/OQ), software designed for 21 CFR Part 11 compliance helps Symphony X users meet and exceed regulatory standards.
IQ/OQ support services verify and document that your instrument is supplied, installed, and operating according to GPT specifications. GPT-certified field service engineers inspect critical components, ensure that your systems function properly and reproducibly during automated synthesis operations, and provide documentation to help you meet regulatory requirements.
21 CFR part 11合规性和IQ/OQ
通过合格人员的培训,全面的质量管理体系,以及包括安装确认和操作确认(IQ/OQ)在内的适当文档,为21 CFR Part 11合规性设计的软件,有助于Symphony X用户满足并超越监管标准。
IQ/OQ支持服务验证并记录您的仪器是否按照GPT规范提供、安装和运行。 gpt认证的现场服务工程师检查关键部件,确保您的系统在自动化合成操作过程中正常运行并可重复使用,并提供文档帮助您满足法规要求。

User Management
用户管理
Administrators can configure passwords by defining password length, complexity, frequency of change, and exclusion of used passwords. Administrators may also define the maximum number of failed login attempts. After a specified (and configurable) period of inactivity, users are automatically logged out and must re-enter a password to log in to the software.
At a minimum, a password is required when creating a new or editing an existing program or sequence file. Beyond this, more stringent controls can be assigned by administrators.
管理员可以通过定义密码长度、复杂度、修改频率和已使用密码的排除情况来配置密码。 管理员还可以定义登录失败的最大次数。 在指定的(可配置的)不活动时间后,用户将自动注销,必须重新输入密码才能登录软件。
创建新程序或编辑现有程序或序列文件时,至少需要密码。 除此之外,管理员还可以分配更严格的控制。
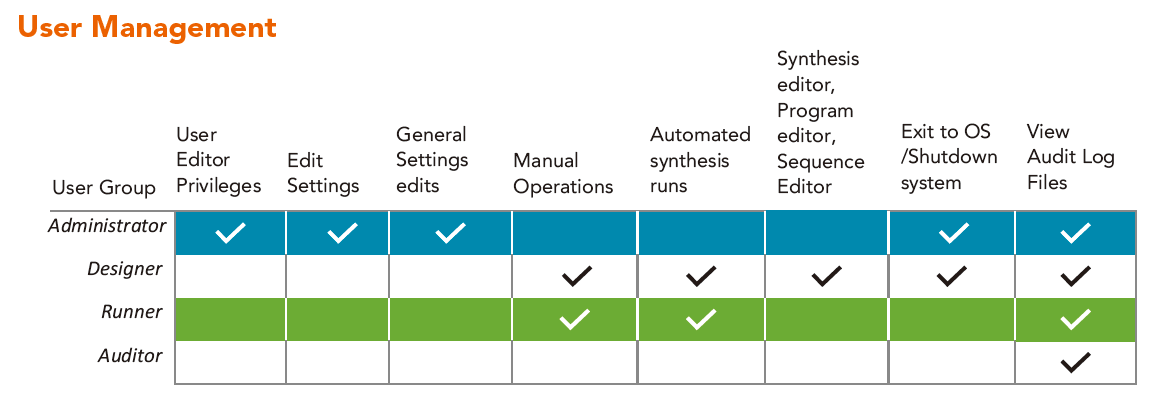
Audit Trail
An audit trail is a time-stamped, modification-protected electronic data file detailing all system events and record modifications. Symphony X software allows a user or auditor to view synthesis run reports, instrument activity log files, user activity log files, and software log files. It is possible to export and print the full contents of all records including the audit trail.
Detailed records of user and software activity as well as data from internal sensors monitoring fluid deliveries, heating, etc. also make these files useful diagnostics for instrument performance and troubleshooting.
审计跟踪
审计追踪是一个有时间戳和修改保护的电子数据文件,详细说明了所有系统事件和记录修改。 Symphony X软件允许用户或审核员查看综合运行报告、仪器活动日志文件、用户活动日志文件和软件日志文件。 可以导出和打印所有记录的完整内容,包括审计跟踪。
用户和软件活动的详细记录以及内部传感器监测流体输送、加热等数据,也使这些文件对仪器性能和故障排除有用的诊断。
Data Integrity
Synthesis program, cleavage, and sequence files are protected and encrypted by document passwords and recorded as digitally signed PDFs using PDFTron technology.
A backup, archiving and recovery strategy is necessary in order to ensure that the reconstruction of data is achievable in the unexpected event of data loss. Symphony X software allows users to create a backup (restore point) on local or external drives, from which they can effectively restore the software.
数据完整性
合成程序、解法和序列文件由文档密码保护和加密,并使用PDFTron技术记录为数字签名的pdf文件。
备份、归档和恢复策略是必要的,以确保在数据丢失的意外事件中可以实现数据重构。 Symphony X软件允许用户在本地或外部驱动器上创建备份(恢复点),从而有效地恢复软件。
Electronic Signatures
Electronic signatures function in the same fashion as hand-written signatures. To meet FDA requirements regarding the use of electronic signatures, the software must employ at least two distinct identification components such as an identification code and password. When a new user is added to the system, they are confirmed to have a unique username, username-password combination, email, and legal name that is used for Digital Signatures.
Completed synthesis runs are signed off by the user who initiated the synthesis. This is manifested in the synthesis run report as a digital signature. Signed synthesis reports contain the printed name of the signer, date, time when the signature was executed, and the reason for signing.
电子签名
电子签名的功能与手写签名相同。 为了满足FDA关于使用电子签名的要求,软件必须使用至少两个不同的识别组件,如识别代码和密码。 当添加新用户时,需要确认新用户的用户名、用户名和密码的组合、电子邮件和“数字签名”的合法名称是唯一的。
完成的合成运行由发起合成的用户签字。 这在综合运行报告中表现为数字签名。 签名的综合报告包含签名者的打印姓名、日期、执行签名的时间和签署的原因。
Audit Support Documentation
In addition to training records, feature, and user guides, an audit support document can be provided that explains in detail how individual software features align with the specific requirements outlined in 21 CFR Part 11.
审计支持文档
除了培训记录、特性和用户指南之外,还可以提供审计支持文档,详细解释单个软件特性如何与21 CFR第11部分中概述的特定需求保持一致。
Symphony X
SYSTEM SPECIFICATIONS系统规范
Number of reaction vessels:24 (12 with pre-activation)
反应容器数量:24个(预激活12个)
Synthesis scale range:0.005* mmol - 24 mmol (up to ~2 g of resin per RV) *minimum deliveries 1 mL or 0.5 mL with Single-Shot
合成规模范围:0.005* mmol - 24 mmol(每RV可达2 g树脂)*最小交付1 mL或0.5 mL单次注射
Reaction vessel volume:Plastic, disposable - 10 mL, 45 mL;Borosilicate glass - 10 mL, 40 mL
反应容器体积:塑料,一次性- 10ml, 45ml;硼硅酸盐玻璃- 10ml, 40ml
Number of solvent positions:1 primary solvent, 20 L capacity. Other sizes available
7 user-defined solvent positions, 0.5 L - 4 L capacities. Other sizes available
溶剂位置数:一次溶剂1个,容量20l。 其他尺寸
7个用户定义的溶剂位置,0.5 L - 4l容量。 其他尺寸
Number of amino acid positions:Up to 40 amino acid positions 10 mL, 120 mL and 400 mL bottles
氨基酸位置数量:多达40个氨基酸位置10毫升,120毫升和400毫升瓶
Chemistries supported:Fmoc, t-Boc, organic, peptoid, combinatorial, branched, PNA
支持的化学:Fmoc, t-Boc,有机,类肽,组合,分支,PNA
Activation:DIC/HOBt, DIC/OxymaPure, HBTU, HATU, HCTU, TBTU, PyBOP and others
激活:DIC/HOBt、DIC/OxymaPure、HBTU、HATU、HCTU、TBTU、PyBOP等
Throughput:User defined
处理量:用户定义的
Fluid transfer method:Positive pressure with nitrogen
流体输送方法:正压氮气输送
Agitation method:Nitrogen bubbling and/or oscillation mixing can be adjustable and programmable
搅拌方式:氮气鼓泡和/或振荡搅拌可调可编程
Otional heating method:Induction heating with IR pyrometer temperature sensinge
加热方式:感应加热,采用红外高温计测温
Cleavage:Automatic, programmable (for Fmoc chemistry only)
切割:自动,可编程(仅适用于Fmoc化学)
Waste container:(2) 20 L D.O.T rated containers with over-flow sensor in cap
废物容器:(2)20升的运输量额定容器,瓶盖上有溢出传感器
Reporting:Real-time log file updated for every instrument function, print to external file, pull up on screen or printer
报告:实时日志文件更新每一个仪器功能,打印到外部文件,拉上屏幕或打印机
Power:115 V/60 Hz or 230 V/50 Hz
功率:115 V/60 Hz or 230 V/50 Hz
Dimensions:45”W, 29”D, 66”H (114 cm W, 74 cm D, 168 cm H)
尺寸:45”W, 29”D, 66”H (114 cm W, 74 cm D, 168 cm H)
Weight:550 lbs. (250 kg)
重量:550磅(250公斤)
Warranty:One year, parts and labor
保修:一年,包括配件和人工
Optional:Infrared (IR) Heating UV monitoring
可选:红外(IR)加热紫外监测